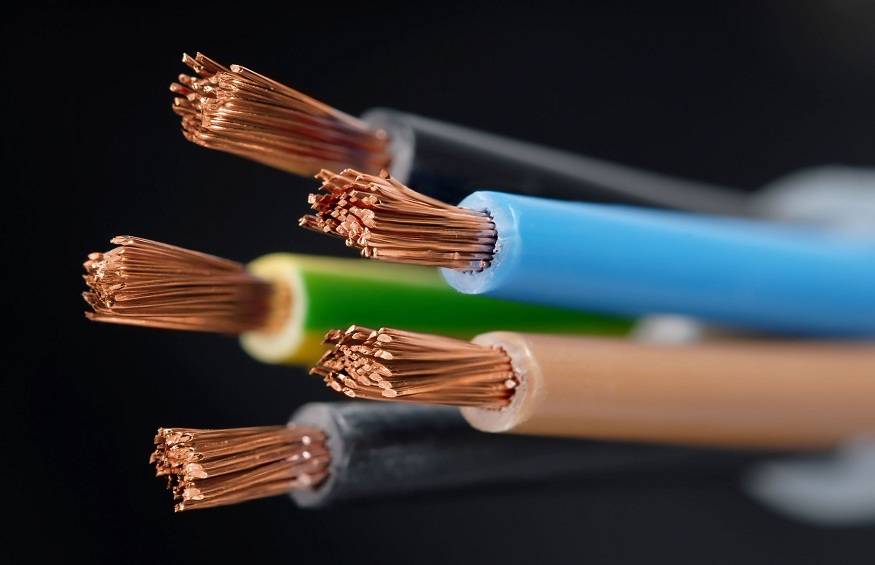
Copper – Good Conductor of Electricity
Copper is a good conductor of electricity, and because of this, it is commonly used in many electrical applications, including home wiring. Due to their high thermal conductivity, copper and several of its alloys are excellent heat exchanger materials for air conditioning and refrigeration systems. Additionally, copper alloys are utilised in plumbing and heating applications as tubes, pipelines, and fittings.
To change the properties of copper, iron is alloyed with zinc, tin, aluminium, nickel and other elements. Brass is the usual name for copper that has been alloyed with zinc. The precise ratio of copper and zinc in brass determines its mechanical qualities. Copper and tin are combined to make bronze. In addition, copper and aluminium can be alloyed to form aluminium bronze. Additionally, hydraulic brake lines, pumps, and screws all use copper and its alloys. The majority of the copper that is extracted is used in electrical, electronic and building construction items.
Does Copper Conduct Heat More Than Iron?
The copper conducts heat more quickly than the iron if we take two wires of identical size and length, one made of copper and the other of iron, and set one end of each in a flame. Compared to iron, copper transmits heat better. A piece of wood can be handled in the same way while it burns to ashes, while a rod of glass can be melted within an inch of the hands holding it without burning them. Thus, we discover that each solid can conduct heat at a special rate.
Gases conduct it to the degree that is even lower than that of liquids. Good conductors are defined as having a high thermal conductivity, while poor conductors or non-conductors do not. What is a good conductor? Those materials with few electrons in the outer orbit are good conductors. Copper, silver and gold are three common metals that are good conductors, and each has one electron in the outer orbit. Due to their ease of transition from one atom to another, these electrons are regarded as free electrons.
Even though mercury and aluminium each have two and three electrons in their outermost electron shells, respectively, these metallic compounds are nonetheless effective conductors.
The Future of Glycerol
Substances whose aqueous solution does not conduct electricity are called non-electrolytes. They do not conduct electricity in the fused state. Solutions of cane sugar, alcohol, glycerine, glucose and urea are examples of non-electrolytes.
The name glycerol is derived from the Greek word for “sweet.” The terms glycerin, glycerine, and glycerol tend to be used interchangeably in the literature. Glycerin or glycerine, on the other hand, generally refers to a commercial solution of glycerol in water, the main component of which is glycerol. Glycerin uses include medical and pharmaceutical preparations, mainly as a means of improving smoothness, providing lubrication and as a humectant, that is, as a hygroscopic substance that keeps the preparation moist.
Glycerol controls water activity, provides humectants, maintains texture and extends shelf life in a variety of applications. Based on the same cause of hyperosmotic action, it is commonly employed in expectorants and cough syrups as a laxative. Glycerol is used as a solvent and lubricant in a wide range of personal care products, including toothpaste, where its superior solubility and flavour give it an advantage over sorbitol.


